$25 Off First Wellness Exam!
(631) 473-0942
New clients will receive $25 off their first wellness exam! Mention this coupon to receive your discount.
*Discount forfeited if you miss your appointment without calling us ahead of time to reschedule.
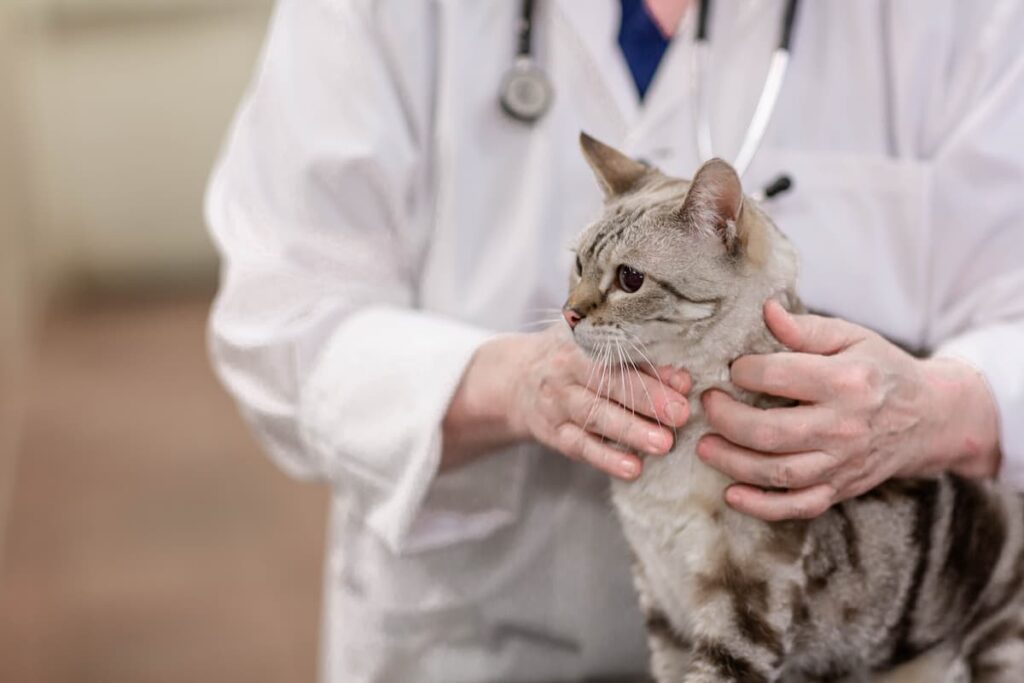
Complete Wellness Care in Port Jefferson, NY
Routine wellness exams are a critical part of your pet’s healthcare routine to maintain their well-being and detect any medical issues before they progress. During your pet’s wellness exam, we will conduct an in-depth nose-to-tail examination and advise you on all aspects of your pet's care.
Comprehensive Care
Preventative Services
Surgical Services
Diagnostics


Talk about caring, loving vet's and staff. I know that when I take my boy to the vets, he is getting the best of care. I am so trusting in their care and standards, that they are the only place that I board my dog. He comes back happy, healthy and well cared for. I absolutely love it here.
Melissa C.

Highly Rated on Google Reviews!
 80+ Reviews
80+ Reviews
Pet Dentistry in Port Jefferson, NY
Just like humans, pets require regular oral care to keep them from developing dental disease. Improper care of your pet's teeth can become very painful and eventually lead to serious illness. Our team is here to help protect your pet’s health and teach you the easy ways you can keep their teeth clean and healthy.
Preventative Care
Oral Exams
Teeth Cleanings
Extractions
Our Story
The Countryside Animal Hospital's team has been proudly serving the Port Jefferson, NY community and surrounding areas since 1958. We understand your pet is part of your family and we will do everything we can to ensure they live a long and healthy life. Our team stays up to date on modern medicine so we can ensure your pet receives the best care possible. We look forward to becoming your partner in your pet’s healthcare.
Book an Appointment